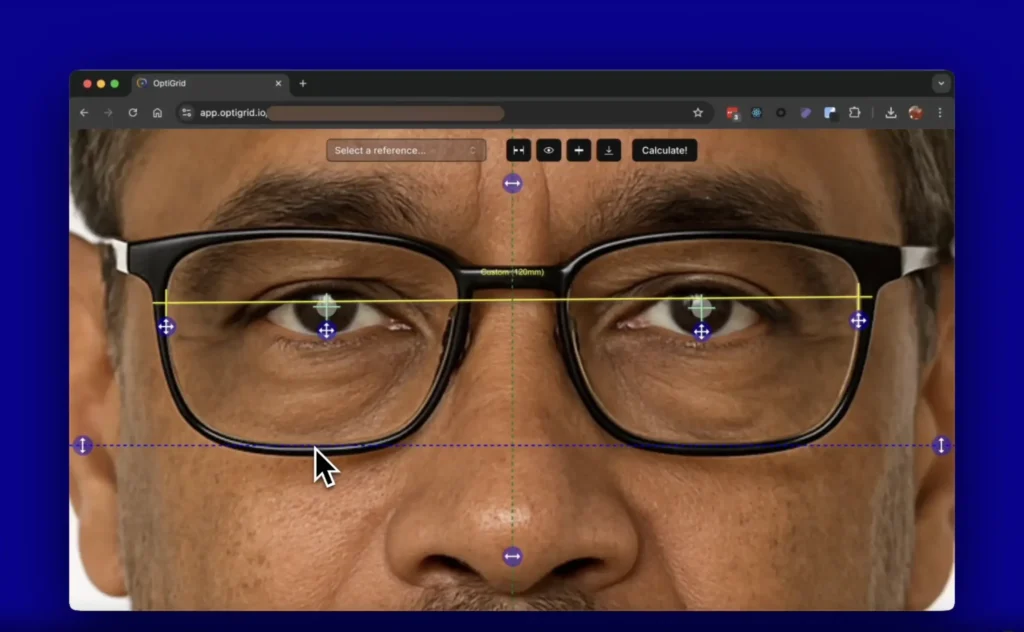
In eye care and eyewear dispensing, pupillary distance (PD) is a small measurement with a big impact: it helps center lenses so patients see comfortably and prescriptions perform as expected. Digital PD rulers are one of several newer tools that aim to make PD measurement faster, more repeatable, and easier to capture in-person or remotely. This article covers what people mean by “digital PD rulers,” how they fit into everyday practice, what we know (and don’t know) about accuracy, where broader tech trends like computer vision and AI show up, how large clinical datasets like the IRIS Registry fit into the bigger picture of eye care, and the practical challenges that come with adopting digital tools.
Key Takeaways
- Digital PD tools can make measurement quicker and more consistent, but results still depend on good lighting, positioning, and calibration.
- In clinics and optical shops, digital options can improve workflow and reduce re-measurements, especially when staff are trained and the process is standardized.
- Computer vision is already part of many PD tools today; “AI” matters most when it improves detection reliability and quality checks—not when it’s used as a buzzword.
- The IRIS Registry is a major ophthalmology dataset used for research and real-world evidence, but it’s not what makes a PD tool accurate (PD accuracy comes from imaging and measurement methodology).
- Adoption challenges are real: trust, training, privacy, and access all need to be handled thoughtfully for digital PD measurement to scale.
Understanding the Digital PD Ruler

The Evolution of Pupillary Distance Measurement
The way we measure pupillary distance (PD) has changed over time—mostly because eyewear fitting has changed. In a traditional optical setting, PD is often measured with a manual ruler or a pupillometer. Those methods can work well, but they also rely on good technique and consistent positioning.
Digital PD tools showed up as more dispensing moved online and as clinics looked for ways to standardize measurements across staff and locations. The goal is simple: make it easier to get a usable PD quickly, and make the result less dependent on who is holding the ruler that day.
A digital PD tool can reduce the “human wiggle room” in the process, but it still needs a clean capture and a consistent workflow to be dependable.
With the introduction of devices like the Optogrid, more businesses are exploring photo-based workflows to support remote eyewear sales and reduce friction when a patient can’t be measured in-person. Read how Optogrid supports optical measurement workflows for eyewear businesses and optometry teams.
How Digital PD Rulers Work
Digital PD rulers are designed to estimate the distance between pupils using a camera (or a sensor) plus software. Most approaches boil down to two jobs: (1) detect facial/eye landmarks reliably, and (2) convert pixels into millimeters using a known reference or a calibrated setup.
The process typically involves the following steps:
- The user positions themselves in front of the digital PD ruler’s camera.
- The device prompts the user to look at a specific point to ensure proper alignment.
- The camera captures an image or video of the user’s face.
- Software analyzes the image to identify the pupils (or related landmarks) and estimate the distance between them.
- The PD measurement is displayed to the user, often within seconds, sometimes with a quality/retake warning.
The best digital PD workflows don’t just output a number—they also help you capture a “good enough” photo so the number is worth trusting.
Compared to a manual ruler, the advantage is consistency and speed. The tradeoff is that image-based tools are sensitive to positioning, camera quality, lighting, and whether the method includes a reliable scaling reference.
Accuracy and Reliability of Digital PD Tools
Accuracy is the part people care about most, and it’s also where marketing often gets ahead of reality. Studies comparing app-based interpupillary distance (IPD/PD) measurements to pupillometers and ruler methods show that results can be close, but differences do happen—and they depend on the device, the app, and how the measurement is performed (distance vs near, alignment, and user guidance).
In practice, digital PD tools tend to do better when they include clear capture instructions and some form of calibration or reference. The most useful question for an optical business isn’t “Is it perfect?” but “Is it consistent enough to reduce remakes and back-and-forth, and do we have a fallback when the capture is poor?”
Digital PD measurement can be reliable when the capture conditions are controlled, but any workflow needs a plan for retakes, verification, and edge cases (kids, strong head turn, heavy brow, glare, or low light).
That’s why ongoing validation matters. If you’re adopting a digital tool, treat it like any other measurement device: test it against your baseline method, document when it fails, and train staff on when to re-capture or switch methods.
The Integration of Digital PD Rulers in Clinical Practice
Adoption by Eye Care Professionals
Digital PD tools are increasingly common in optometric and ophthalmologic environments, but adoption isn’t uniform. Many practices try them for practical reasons: reducing chair time, supporting remote orders, and making measurements more repeatable across staff. Innovations in remote pupillary distance measurement often focus on better guidance during capture and better handling of real-world variability (lighting, pose, and device differences).
The adoption rate varies by region and practice size, with larger practices and urban centers often moving faster. Here’s a snapshot of the current landscape:
- Urban practices: Higher adoption due to greater access to tools and higher demand for remote-friendly workflows
- Rural practices: Gradual adoption as tools become easier to deploy and support improves
- University-affiliated clinics: Often early adopters when the tool supports research or standardized protocols
- Independent optometrists: Adoption varies; many try digital tools when it helps them compete on convenience without losing quality
For most clinics, digital PD tools aren’t about “going high-tech.” They’re about consistency, throughput, and fewer measurement-related issues downstream.
Some practitioners remain hesitant, usually due to cost, training time, or worry about inconsistent captures. Those concerns are fair. The best rollouts start small: define your workflow, train staff, measure outcomes, and only then scale it across the practice.
Impact on Patient Experience
Digital PD tools can improve the patient experience when they reduce friction. Instead of repeating measurements or struggling with a manual ruler, many patients get a faster, more guided process—especially in retail settings or remote workflows.
- Ease of use: Good tools guide the patient clearly and flag bad captures.
- Speed: Measurements can be captured quickly once the workflow is familiar.
- Comfort: Many approaches are non-contact, which some patients prefer.
When digital capture is paired with a clean workflow (intake, verification, and documentation), it can reduce wait time and cut down on repeated measurements.
Accuracy still matters most. A smoother experience isn’t helpful if it increases remake rates. The goal is to make the process easier while keeping the measurement trustworthy.
Case Studies: Success Stories in Clinical Settings
Real-world “success stories” for digital PD measurement usually look like this: fewer measurement redo’s, clearer documentation, and smoother workflows when a patient is ordering eyewear remotely. Some clinics also report fewer post-dispensing adjustments tied to measurement issues—especially when the digital workflow includes verification steps.
- Practices using a standardized digital workflow often report more consistent measurements across staff.
- Some clinics find digital capture speeds up intake, as long as staff know when to retake an image.
- Digital tools can help patient education by making the measurement process easier to explain and repeat.
Digital PD tools work best when they’re part of a process: capture → quality check → confirm → document. That’s what keeps convenience from turning into callbacks.
It’s still worth tracking long-term outcomes: remake rate, returns, and how often staff need to switch to a fallback method. That data will tell you whether the tool is helping or just moving work around.
Technological Advancements in Eye Care
From Manual to Digital: A Technological Leap
The move from manual to digital measurement in eyewear is mostly about standardization. A manual ruler can be accurate in skilled hands, but results vary more across staff and settings. Digital tools aim to reduce that variation and make it easier to support remote eyewear orders and PPE eyewear programs. The digital PD ruler can simplify measurement capture, improve documentation, and reduce errors—when implemented with training and clear quality checks.
Digital measurement doesn’t replace clinical judgment. It’s a way to make the measurement step more repeatable and easier to audit when something looks off.
Overall, this is less about “new gadgets” and more about better processes. The practices that see benefits are usually the ones that treat measurement as a workflow, not a one-off task.
The Role of AI and Machine Learning
AI and machine learning show up in eye care in a few different places. For PD measurement tools, the most practical use is computer vision: detecting landmarks reliably, handling imperfect captures, and flagging low-quality images. In other parts of eye care (like imaging and screening), AI can help interpret patterns in retinal scans or other clinical data.
- Automated landmark detection can make PD capture more consistent in real-world conditions.
- Quality checks can warn users when lighting, pose, or glare makes a measurement unreliable.
- In medical imaging, deep learning can assist clinicians by highlighting patterns that may deserve attention (with appropriate validation and oversight).
AI is helpful when it improves reliability and catches bad inputs. If it can’t explain why a capture is “good,” it won’t earn trust in a clinic.
As AI tools mature, accountability matters. Practices need clarity on how a tool was validated, what conditions affect performance, and what the recommended fallback is when the capture is questionable.
Future Trends in Ophthalmic Biometrics
Ophthalmic biometrics will keep evolving, but not every trend matters equally for eyewear dispensing. In the eyewear world, the practical “next steps” are better remote capture guidance, more consistent calibration methods, and clearer quality scoring so staff know when to trust a result. On the clinical side, biometrics and imaging will continue to benefit from better sensors and more robust analytics.
There’s also growing interest in combining multiple signals—face geometry, gaze direction, and capture quality—to make measurements more stable across different phones and environments. The most useful innovation will be the one that reduces rework without lowering standards.
The future isn’t just “more data.” It’s better capture quality, clearer validation, and fewer edge cases slipping through unnoticed.
We’ll likely see more 3D-aware approaches and multi-sensor techniques over time, but adoption will depend on cost, ease of use, and whether the tools are clearly validated for real dispensing scenarios.
The Role of the IRIS Registry in Advancing Eye Care
Harnessing Big Data for Ophthalmology
The IRIS Registry (Intelligent Research in Sight) is a major ophthalmology clinical registry managed by the American Academy of Ophthalmology. It aggregates de-identified data used for research, quality improvement, and real-world evidence in eye care. While it doesn’t validate PD rulers directly, it does reflect a broader trend: eye care is becoming more data-driven, and tools are increasingly expected to show evidence and measurable outcomes.
- More consistent care pathways through benchmarking and outcomes tracking
- Better visibility into treatment patterns and variations across settings
- Operational insights that can support workflow improvements
- Research foundations for analytics and (in some contexts) AI-assisted decision support
For eyewear professionals, the takeaway is simple: data and validation are becoming table stakes. Whether it’s a clinical tool or a measurement workflow, the “show your work” expectation is rising.
Large registries don’t replace clinical trials, but they can reveal real-world patterns that are hard to see in smaller datasets.
Case Analysis: IRIS Registry Insights
The IRIS Registry has supported studies that compare real-world outcomes to results seen in controlled trials. For example, researchers have used IRIS data to emulate outcomes from major retinal clinical trials such as VIEW 1 and VIEW 2, highlighting how real-world evidence can be analyzed at scale. That kind of work strengthens post-market monitoring and helps the field understand how treatments perform outside ideal trial conditions.
Registry-based research is most useful when it’s clear about data quality, missing data, and how closely the real-world population matches the trial population.
IRIS insights have also been used to understand shifts in eye care delivery (including changes during the COVID-19 period) and to identify gaps and variations in care. Below is a summary of common research themes supported by large registry datasets:
- Real-world evidence studies for retina and glaucoma
- Approaches to handling missing data
- Methods for reducing bias in observational analyses
- Annual reporting and benchmarking of eye care trends
- Care delivery changes during major disruptions (e.g., COVID-19)
The IRIS Registry remains a valuable resource for the ophthalmology community, mainly because it helps turn everyday clinical activity into measurable insights.
Collaborations and Innovations Stemming from IRIS Data
Large registries like IRIS can support collaborations across practices, researchers, and data partners. When data quality improves (including better handling of missing demographic fields), the research becomes more reliable and more useful for understanding outcomes across diverse populations.
- Digital health companies and researchers use registry-style datasets to study outcomes and care patterns; PD measurement tools, however, typically depend on imaging workflows and calibration—not registry participation.
- Methodology work (matching techniques, bias reduction, missing data models) improves how confidently people can use real-world data alongside trial results.
When data collaborations work well, they raise the bar for evidence—what gets adopted, what gets reimbursed, and what becomes standard practice.
From an eyewear perspective, this matters because the entire ecosystem is moving toward clearer evidence and better validation. Tools that can’t explain accuracy, limitations, and quality controls will struggle to earn long-term trust.
Challenges and Considerations for Digital PD Adoption

Overcoming Skepticism in the Medical Community
The adoption of digital PD measurement has been mixed. Some practices adopt early because it helps with workflow and remote orders; others wait because they’ve seen inconsistent app results or worry the tool will be used without proper technique. This example from an optometry practice shows how clinics think about bringing digital tools into day-to-day care—but the concerns are still worth taking seriously.
Skepticism often comes down to two things: (1) “Will this be accurate enough in real life?” and (2) “Will my staff use it correctly?” The best way to reduce skepticism is not with promises, but with clear validation, staff training, and defined rules for retakes and fallbacks.
- Clear validation data builds trust faster than marketing claims.
- Privacy expectations vary by region and patient population, so communication needs to be tailored.
- Global adoption is easier when tools are transparent about what they store, what they don’t, and why.
Digital measurement earns trust when it’s easy to explain, easy to repeat, and honest about when it might be wrong.
Navigating Regulatory and Privacy Concerns
Digital PD workflows often involve face images or biometric-like data, which raises real privacy and security questions. If a tool captures photos, stores them, or uses them to compute measurements, it needs clear policies and strong security practices.
- Transparent data protection measures
- Access control mechanisms
- Clear communication with patients
Trust is fragile here. Patients usually accept digital capture when they understand what’s being collected, how long it’s kept, and who can access it.
Convenience is great, but it can’t come at the expense of privacy. Clear consent and clear retention policies matter.
Any clinic or eyewear business adopting digital PD tools should confirm applicable requirements (local health privacy laws, consumer privacy rules, and vendor contracts) and ensure staff know how to handle patient data safely.
Ensuring Equitable Access to Digital Eye Care Tools
Digital PD measurement can improve access—especially for remote patients—but only if the tools are practical across different devices and communities. Equity isn’t only about price; it’s also about language, guidance, and the ability to capture a usable image in everyday conditions.
- Awareness: Patients and staff need to understand what PD is and why a good capture matters.
- Affordability: Pricing and hardware requirements should match the reality of small practices and budget-conscious buyers.
- Accessibility: Tools should work in varied environments (not just perfect lighting with the latest phone).
- Training: Short, practical training and clear “when to retake” guidance make adoption smoother everywhere.
The hard part isn’t building the tool—it’s building a workflow that works for different people, different devices, and imperfect conditions.
While the adoption of Digital PD (pupillary distance) tools brings benefits, it also requires practical support: clear capture steps, a verification method, and an option to fall back to manual measurement when needed. To explore a photo-based workflow for optical measurements, access our app and explore OPTOGRID. Sign in to test how it fits into your dispensing process.
Conclusion
Digital PD rulers and camera-based PD tools can make measurement faster and more consistent, especially when you’re supporting remote eyewear orders. The upside is real: better workflow, clearer documentation, and fewer measurement do-overs—when the capture quality is good and the team follows a standard process. At the same time, accuracy isn’t automatic. Tools should be validated, staff should be trained, and patients should be guided clearly. Broader trends in digital health and data (including large datasets like the IRIS Registry) show that eye care is moving toward more evidence-driven tools and higher expectations around transparency. In that environment, the digital PD tools that win long-term trust will be the ones that are honest about limitations and strong on quality control.
Frequently Asked Questions
What is a Digital PD Ruler?
A Digital PD (Pupillary Distance) Ruler is a tool used to measure the distance between the centers of the pupils in each eye. This measurement helps center eyeglass lenses correctly, and many tools now estimate it using a camera plus software.
How does a Digital PD Ruler improve clinical practice?
Digital PD tools can speed up measurement and reduce variation between staff, especially when the workflow includes guidance and quality checks. In many settings, this improves documentation and can reduce back-and-forth with patients when ordering eyewear remotely.
What role does AI play in the evolution of eye care tools?
In PD measurement, AI typically shows up as computer vision: detecting eye landmarks, scoring capture quality, and reducing errors from poor alignment or lighting. In other areas of eye care, AI can assist with screening and imaging analysis (with validation and clinical oversight).
How does the IRIS Registry contribute to advancements in eye care?
The IRIS (Intelligent Research in Sight) Registry is a major ophthalmology clinical registry managed by the American Academy of Ophthalmology. It supports research and real-world evidence by enabling analysis of de-identified ophthalmic data at scale.
What are the challenges in adopting Digital PD tools?
Challenges include building trust in accuracy, training staff, handling privacy and data retention appropriately, and ensuring the workflow works for different patients and devices. A good rollout also includes a fallback method when capture conditions aren’t good.
Can Digital PD Rulers be used remotely, and how has the pandemic affected their use?
Yes. Many digital PD tools support remote capture, which is useful for online eyewear orders and telehealth-adjacent workflows. The COVID-19 period increased interest in remote-friendly processes, but quality control remains important: remote capture needs clear guidance and a way to verify or redo measurements when needed.

I am a seasoned software engineer with over two decades of experience and a deep-rooted background in the optical industry, thanks to a family business. Driven by a passion for developing impactful software solutions, I pride myself on being a dedicated problem solver who strives to transform challenges into opportunities for innovation.